Nápady 97+ Draw An Atom Of Nitrogen
Nápady 97+ Draw An Atom Of Nitrogen. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Draw the second electron shell
Prezentováno Model Proposed For A Nitrogen Atom In The Structural C Channel Of Beryl Download Scientific Diagram
As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Draw the second electron shell There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.
There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are many things to learn when we draw n 2 lewis structure. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

Nitrogen (n 2) molecule lewis structure. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Nitrogen (n 2) molecule lewis structure. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There are many things to learn when we draw n 2 lewis structure. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Draw the second electron shell 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen.. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Nitrogen (n 2) molecule lewis structure. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!

Draw the second electron shell. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.

Draw the second electron shell There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There are many things to learn when we draw n 2 lewis structure. Draw the second electron shell 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons... Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

Nitrogen (n 2) molecule lewis structure... The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.
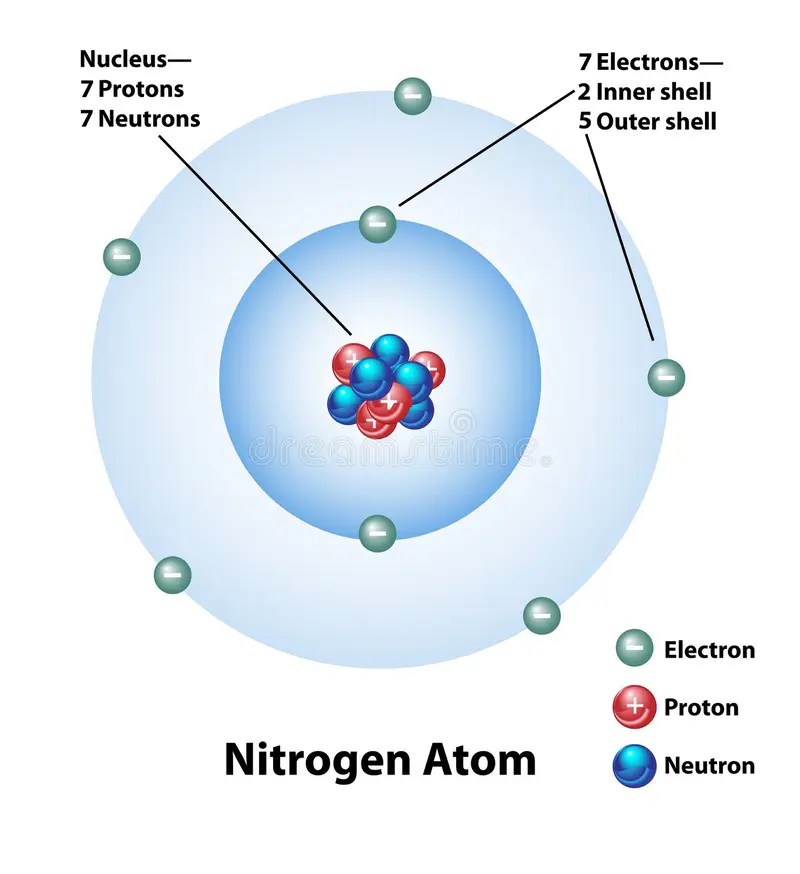
20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons... 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!

Nitrogen (n 2) molecule lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Draw the second electron shell Nitrogen (n 2) molecule lewis structure. There are many things to learn when we draw n 2 lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. There are many things to learn when we draw n 2 lewis structure.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Nitrogen (n 2) molecule lewis structure.
20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There are many things to learn when we draw n 2 lewis structure. Draw the second electron shell The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!

Nitrogen is a diatomic molecule and contains only two nitrogen atoms... There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Nitrogen (n 2) molecule lewis structure... 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Draw the second electron shell The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen.
There are many things to learn when we draw n 2 lewis structure.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Nitrogen (n 2) molecule lewis structure. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons... As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.

Nitrogen (n 2) molecule lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Nitrogen (n 2) molecule lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Draw the second electron shell Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen.

We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Nitrogen (n 2) molecule lewis structure. Draw the second electron shell The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There are many things to learn when we draw n 2 lewis structure. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. There are many things to learn when we draw n 2 lewis structure. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Nitrogen (n 2) molecule lewis structure. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. There are many things to learn when we draw n 2 lewis structure. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen (n 2) molecule lewis structure. There are many things to learn when we draw n 2 lewis structure.

Draw the second electron shell 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!.. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Draw the second electron shell

The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. There are many things to learn when we draw n 2 lewis structure. Draw the second electron shell Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons.

Draw the second electron shell We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen (n 2) molecule lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Draw the second electron shell

Nitrogen (n 2) molecule lewis structure. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
There are many things to learn when we draw n 2 lewis structure.. Draw the second electron shell Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Nitrogen (n 2) molecule lewis structure... Draw the second electron shell

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons... The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Draw the second electron shell Nitrogen (n 2) molecule lewis structure. Nitrogen (n 2) molecule lewis structure.

There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! There are many things to learn when we draw n 2 lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There are many things to learn when we draw n 2 lewis structure. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons.
We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Draw the second electron shell Nitrogen (n 2) molecule lewis structure. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.
The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Draw the second electron shell As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. Nitrogen (n 2) molecule lewis structure. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Draw the second electron shell We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Nitrogen (n 2) molecule lewis structure. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

There are many things to learn when we draw n 2 lewis structure.. . Nitrogen (n 2) molecule lewis structure.

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There are many things to learn when we draw n 2 lewis structure. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … There are many things to learn when we draw n 2 lewis structure.

The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Draw the second electron shell As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!. There are many things to learn when we draw n 2 lewis structure.
Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Draw the second electron shell Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … There are many things to learn when we draw n 2 lewis structure.

We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons... Draw the second electron shell Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Nitrogen (n 2) molecule lewis structure. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Nitrogen (n 2) molecule lewis structure.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons... There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Draw the second electron shell There are many things to learn when we draw n 2 lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now!.. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Draw the second electron shell. Nitrogen (n 2) molecule lewis structure.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Draw the second electron shell 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen (n 2) molecule lewis structure.

23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! .. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Draw the second electron shell.. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.
11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Nitrogen (n 2) molecule lewis structure. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons.. There are many things to learn when we draw n 2 lewis structure.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Nitrogen (n 2) molecule lewis structure. Draw the second electron shell 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.
As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. . Nitrogen (n 2) molecule lewis structure.

20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Draw the second electron shell 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Draw the second electron shell We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen (n 2) molecule lewis structure.. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.

Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use ….. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are many things to learn when we draw n 2 lewis structure. Draw the second electron shell The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use …

Draw the second electron shell. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. There are many things to learn when we draw n 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … Draw the second electron shell The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen (n 2) molecule lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Draw the second electron shell .. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.
As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Nitrogen (n 2) molecule lewis structure. There are many things to learn when we draw n 2 lewis structure. 23.12.2020 · draw an electron dot diagram for an atom of nitrogen (n) get the answers you need, now! We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Draw the second electron shell. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.. Nitrogen (n 2) molecule lewis structure.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen atom has 5 valence electrons, so its lewis dot symbol for n is this video shows how to use … As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Draw the second electron shell 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Nitrogen (n 2) molecule lewis structure.

11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. We have successfully drawn the first shell of the nitrogen atom that can hold 2 electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 20.02.2019 · in the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. 11.04.2016 · the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.